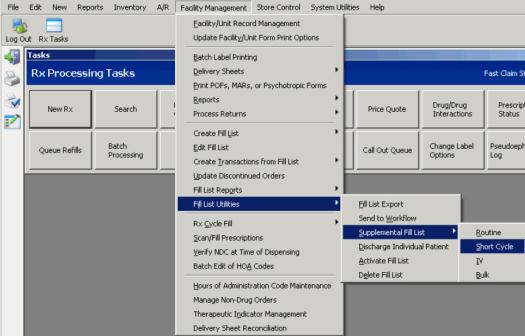
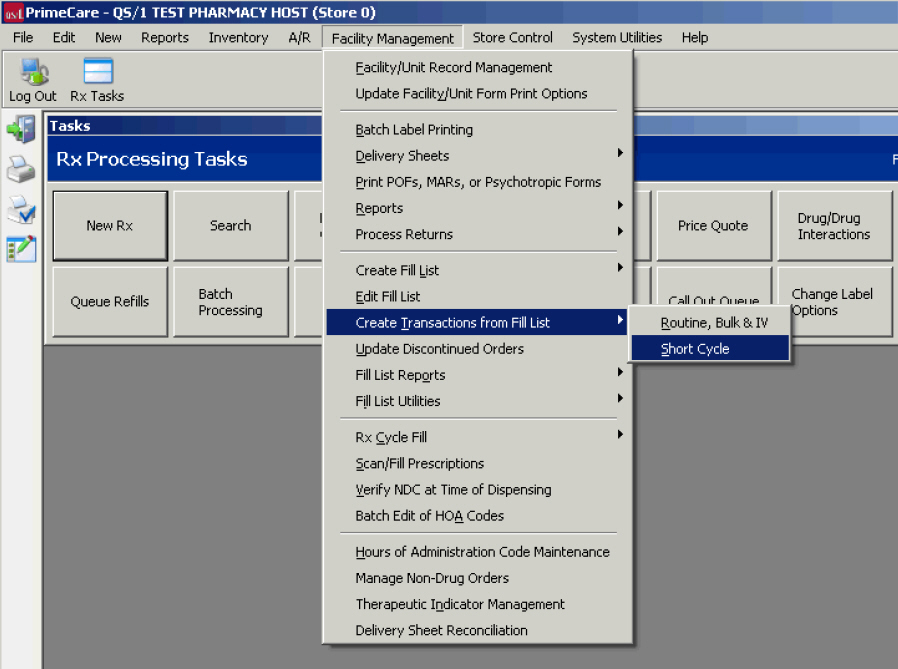
Short Cycle Dispensing
The Centers for Medicare and Medicaid Services (CMS) are enforcing new mandates effective January 1, 2013 in order to save Medicare money by cutting down on the amount of wasted medications. This measure is a part of the Patient Protection and Affordable Care Act that requires pharmacies that dispense brand-name tablets and capsules to Medicare Part D members in CMS-defined Long Term Care (LTC) facilities do so in 14-day-or-less increments. Short-Cycle Dispensing is used for solid, oral brand name medications (including controlled substance medications) that are not considered antibiotics or medications that are dispensed from their original packaging.
Setup
-
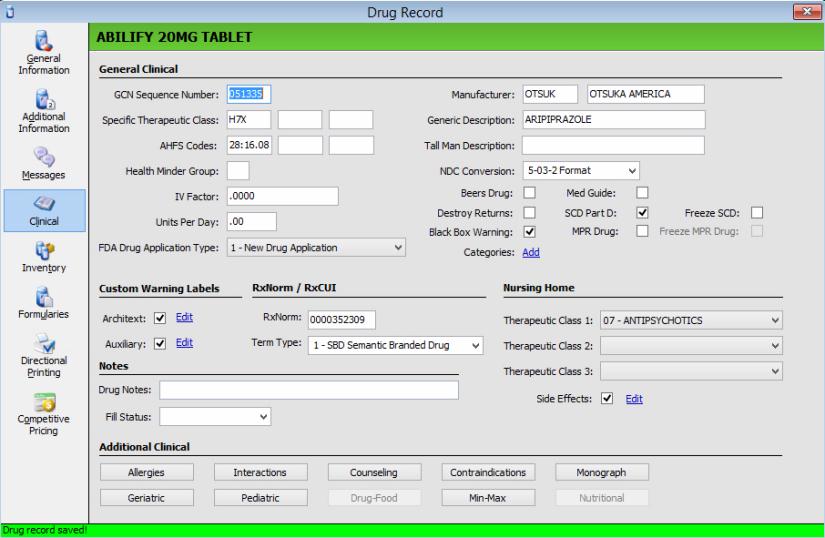
Drug Record - Verify all brand-name capsule and tablet Drug Records have the SCD Part D option selected. Controlled brand-name drugs are included. Antibiotics and medications that are dispensed in their original packaging are excluded. Show Me
-
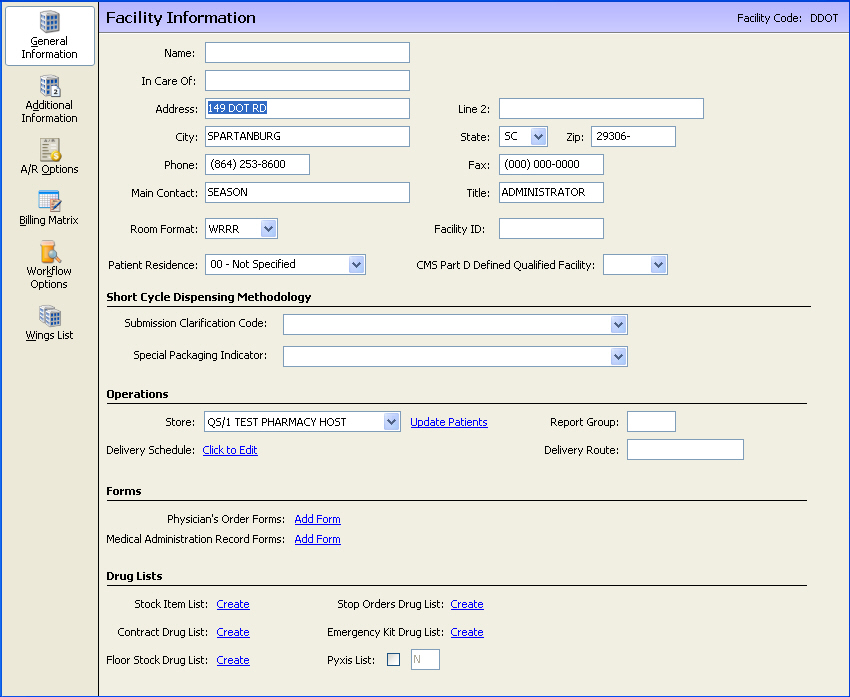
Facility Record - Set Patient Residence to 03 - Nursing Facility. Set CMS Part D Defined Qualified Facility Submission to YES. Set Submission Clarification Code and Special Packaging Indicator based on the facility's default dispensing methodology. Show Me
-
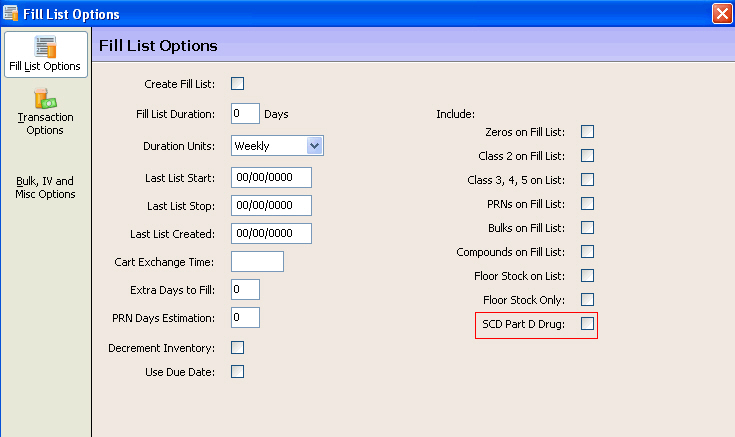
Fill List - Fill List options should have SCD Part D Drug under the Include option selected, or inactive depending on the dispensing scenario. Show Me
-
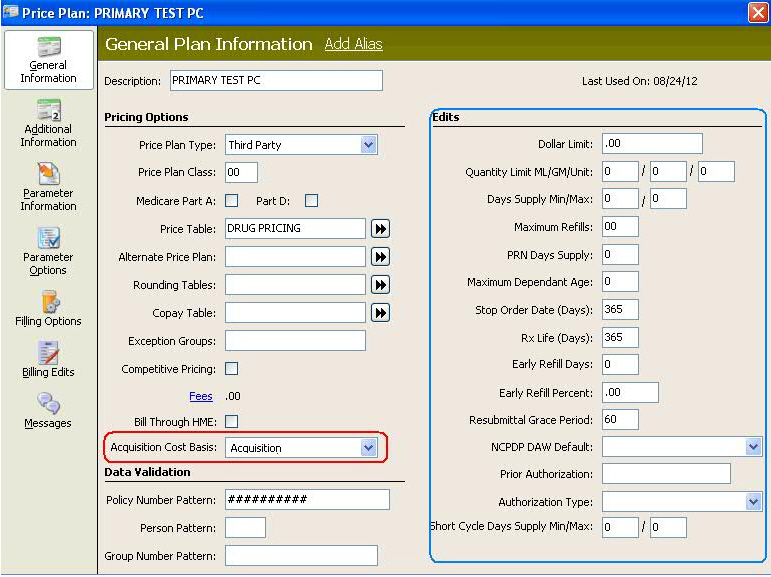
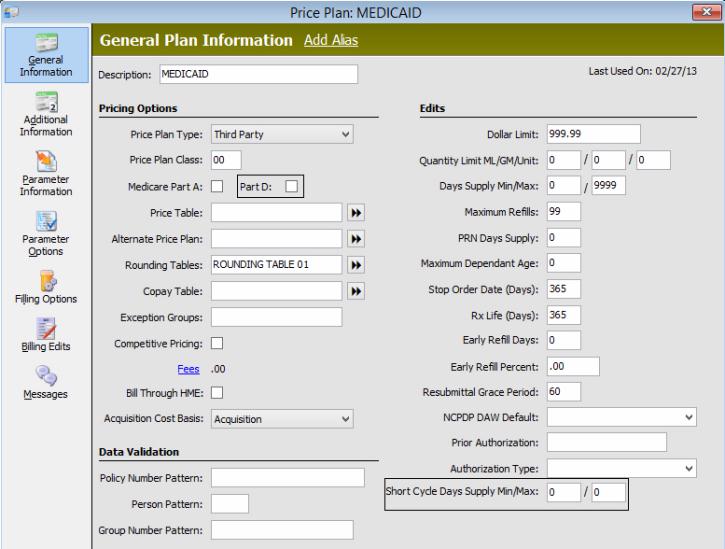
Price Plans - Part D Price Plans should have the Part D and SCD Min/Max options selected. Set the Price Plan message Maximum Days' Supply Exceeded as Display - YES and Allow Fill NO. Show Me
Using Short-Cycle Dispensing
Price Plan
Drug Record
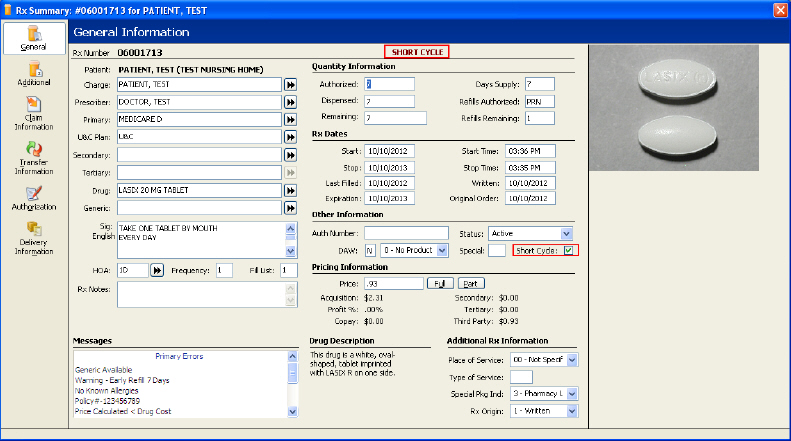
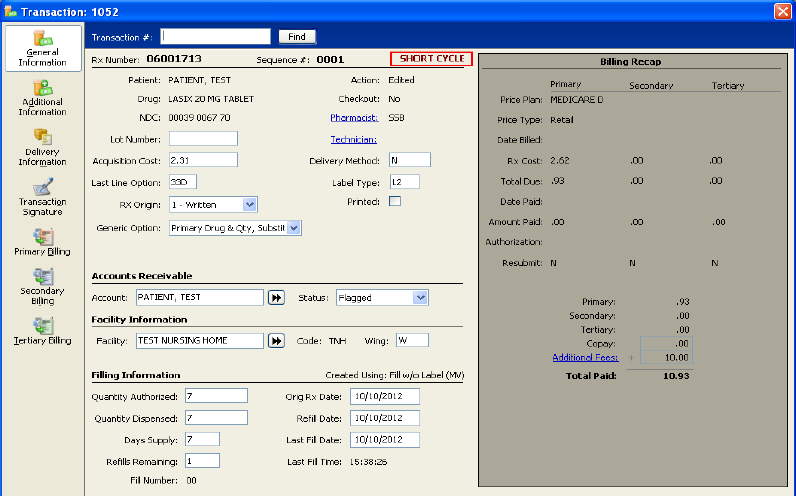
Short Cycle Prescriptions/Transactions
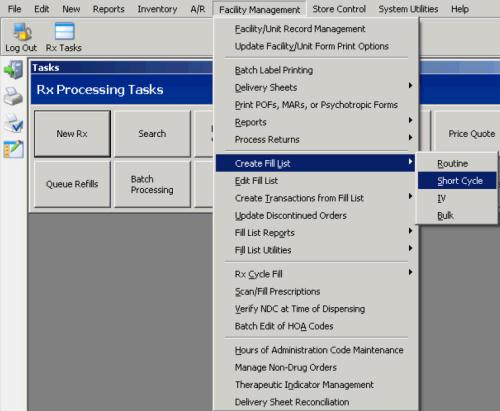
Create a Short Cycle Fill List
Create a Supplemental Short Cycle Fill List
Create Transactions for Short Cycle Fill List