The Centers for Medicare and Medicaid Services (CMS) rule for short cycle dispensing takes effect January 1, 2013. This measure, which is part of the Patient Protection and Affordable Care Act, requires pharmacies that dispense brand tablets and capsules to Medicare Part D members in CMS defined long term care (LTC) facilities do so in 14-day-or-less increments. This mandate is intended to save money for Medicare by cutting down on the amount of wasted medications.
To facilitate short cycle dispensing, Part D Third Party Price Plans, Drug Records and Facility Records must be set up to process dispensing to Medicare Part D patients in long term care facilities.
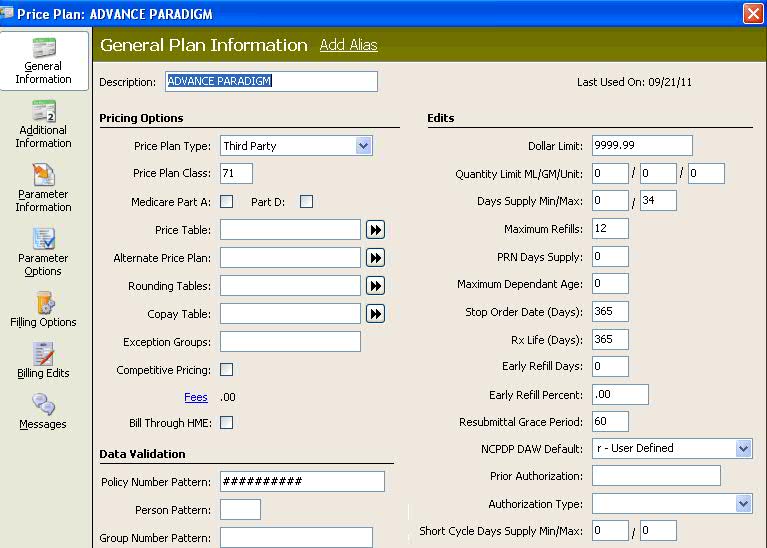
Set a Min/Max Days Supply on the Price Plan, General Information screen based on drug unit. The two fields default = Blank. If a prescription is identified as short-cycle dispensing (SCD) and the Min/Max quantities are not met, error messages display. These messages display when the following conditions are met:
The Drug Record has a SCD Part D Drug set to Y
The Facility Record CMS Defined Part D is set to Y
The Patient Residence = 03 on the Prescription Record
The SCD Part D Brand field must be checked on the Drug Record and Batch Drug Record Clinical screens. This field can be edited by the user and is updated by the following programs:
Update drug data from CD
Update drug data from QS/1 internet updates
Batch drug updates from CD
Price updates from diskette
Price Updates
Update from CMS
During the conversion, this field is set to Y when all the conditions listed below are met and the Specific Therapeutic Class field does not contain a W1. W1 Therapeutic Classes are antibiotics and excluded from SCD. Conditions are:
The FDA drug field is set to 1.
The Generic Indicator field is blank or * (single source or multi-source).
The Drug Unit is either CAP, TAB, CP, TB, CPM, TBD, CMB, TBM or CSM.
The Freeze SCD field is available on the Drug Record and Batch Drug Record Clinical screens. This field is only active when the SCD Part D field is checked. Checking this field prevents the SCD Part D field from being changed during Clinical Updates. This field is available as a Drug File Report Option as DG-Freeze SCD.
The Short Cycle Dispensing Methodology section is located on the Facility Record, General Information screen. The two fields in this section are Submission Clarification Code and Special Packaging Indicator and are only active when the CMS Part D Defined Facility = Yes and the Patient Residence = 03. New codes can be added as needed for either option. These fields are copied to the Prescription and Drug Records. If these fields are not completed during processing, the message Submission Clarification Code and Special Packaging Indicator are required for short cycle billing, displays.
Short Cycle displays in the upper right corner of the Prescription and Transaction screens to indicate a SCD prescription. If a prescription is profiled and a SCD, both Short Cycle and Profiled display. These fields displays when the following qualifications are met:
Price Plan is a Part D
Drug is a SCD Part D
Facility is a CMS Defined Part D Facility
Patient Residence = 03
Short Cycle fields are available as Report Select, Sort and Print Options:
Rx-Short Cycle Disp
Tx-Short Cycle Disp
Return to Facility Management Overview